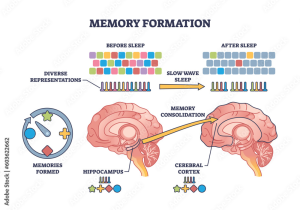
Neurodegenerative disease research is at the forefront of modern neuroscience, unveiling critical insights into disorders like Alzheimer’s disease and Huntington’s disease. As we delve deeper into how microglial cells function as the brain’s immune system, we discover their pivotal role in maintaining neurological health. These immune-like cells not only defend against pathogens but also prune damaged synapses, highlighting the intricate relationship between brain health and immune response. However, when this process malfunctions, it contributes to the progression of neurodegenerative diseases, emphasizing the urgent need for more targeted research. The discoveries made in this domain have the potential to reshape treatment protocols and improve the quality of life for millions affected.
Research into neurodegenerative disorders encompasses a wide range of studies focused on various types of brain diseases that lead to the degeneration of nerve cells over time. This field explores how conditions like Alzheimer’s and Huntington’s not only impact cognitive abilities but also disrupt the overall brain immune system, which includes crucial components such as microglial cells. By investigating these mechanisms, scientists aim to uncover new therapeutic targets that could mitigate symptoms and enhance patient care. Moreover, the intersection of neuroscience and immunology in this research stream is vital for developing innovative treatments that could alter the landscape of care for individuals battling these debilitating conditions. As interest grows in understanding the complexities of brain diseases, collaborative efforts are essential for translating these findings into meaningful clinical advances.
Understanding Microglial Cells in Neurodegenerative Disease Research
Microglial cells play a crucial role in the brain’s immune system, acting as vigilant guardians that patrol for signs of injury or disease. In the realm of neurodegenerative disease research, scientists like Beth Stevens are uncovering the intricate ways in which these cells contribute to conditions such as Alzheimer’s disease and Huntington’s disease. By investigating how microglia engage in synaptic pruning, researchers hope to better understand the mechanisms that lead to neuronal dysfunction and loss. Such insights could be transformative, paving the way for novel therapeutics aimed at preserving cognitive function in affected individuals.
The Stevens Lab’s revolutionary findings highlight how abnormal microglial activity can exacerbate neurodegenerative processes. This exploration is critical, as it lays the groundwork for differentiating between normal and pathological brain immune responses. Enhanced comprehension of microglial dynamics not only aids in the identification of new biomarkers for early detection of these diseases but also contributes to the design of informed treatment strategies, ultimately improving the quality of life for millions suffering from neurodegenerative conditions.
The Transformational Impact of Neurodegenerative Disease Research
Research into neurodegenerative diseases has shifted dramatically over the years, showcasing a transformation in the understanding of these complex disorders. Beth Stevens, backed by NIH funding, exemplifies how curiosity-driven studies can unveil crucial biological pathways. Her work on microglial involvement in synaptic pruning illustrates that foundational research can yield unexpected insights into clinical implications. As scientists investigate the connection between immune responses in the brain and diseases like Alzheimer’s, they are finding that these basic science inquiries can lead to breakthroughs in our comprehension and treatment of such conditions.
Moreover, as Stevens emphasizes, this research is not merely academic; it is a potent means to facilitate clinical advancements. Discoveries made in laboratory settings often catalyze the development of innovative biomarkers and therapies, contributing to a healthcare landscape where neurodegenerative diseases might become manageable or even preventable. The interplay between basic science and medical research is vital, demonstrating that understanding cellular mechanisms in microglia will lead to pathways for novel interventions—potentially changing the trajectory of Alzheimer’s and other neurodegenerative diseases.
The Role of Pruning in Neurodegenerative Disease Progression
Abnormal synaptic pruning by microglial cells has emerged as a significant factor in the progression of neurodegenerative diseases. This process, which is normally beneficial for brain health, can have devastating effects if not regulated properly. During research studies, including those conducted by Stevens, it has been observed that excessive or erroneous pruning can lead to the loss of critical neural connections, contributing to the cognitive decline seen in Alzheimer’s disease. Understanding this abnormality provides a crucial insight into how altering the behavior of microglia could potentially mitigate disease symptoms and progression.
Furthermore, the distinction between normal and aberrant pruning behaviors opens up exciting avenues in neuroscience research. Identifying the triggers for such pathological activity can lead to targeted therapies that restore proper microglial function. By unraveling the complexities of immune cell interactions within the brain, researchers aim to not only halt the progression of diseases like Huntington’s but also enhance neuronal resilience, providing hope to those afflicted by these debilitating conditions.
Advancements in Biomarkers for Alzheimer’s and Other Disorders
The identification of novel biomarkers is a pivotal area in the realm of Alzheimer’s research, greatly influenced by advancements in our understanding of microglial cells. Stevens’ pioneering work underscores how recognizing the patterns of microglial activity could lead to early diagnostic markers for Alzheimer’s disease. Biomarkers derived from these findings have the potential to detect the disease in its nascent stages, facilitating prompt intervention—crucial for effective management of neurodegenerative conditions.
Moreover, these biomarkers not only serve diagnostic purposes but also play a significant role in disease monitoring and assessing the efficacy of new treatments. As researchers continue to explore the relationship between microglial behavior and neurodegeneration, the dream of establishing reliable and accessible markers grows closer to reality. This move towards precision medicine will empower clinicians to tailor interventions based on individual patient profiles, which could dramatically improve the prognosis for those diagnosed with Alzheimer’s or similar disorders.
Neuroscience Research: A Foundation for Future Innovations
Neuroscience research forms the backbone of our understanding of brain-related disorders, including Alzheimer’s and Huntington’s diseases. As researchers like Beth Stevens delve into the biological underpinnings of these conditions, they not only expand the scientific community’s knowledge but also stimulate innovations in therapeutic approaches. The adaptations in research focus, from purely observational studies to more nuanced explorations of microglial functions, exemplify the dynamic nature of neuroscience.
This evolving nature of research signifies a broader cultural shift within the scientific community, emphasizing the importance of curiosity-driven inquiries. Such studies unveil important connections between basic biology and clinical applications, positioning neuroscience as a key player in the quest to combat neurodegenerative diseases. Ultimately, the interplay of innovation and foundational research will catalyze breakthroughs that transform care for millions affected by these conditions, highlighting the critical importance of continued investment in neuroscience research.
Federal Funding as a Catalyst for Neurological Discoveries
Federal funding programs, such as those spearheaded by the National Institutes of Health, play an essential role in propelling discoveries within neurodegenerative disease research. As Stevens highlights, her work benefitted tremendously from the support of federal agencies, allowing for the exploration of microglial functions in a way that would not have been possible otherwise. Such financial backing is critical in fostering an environment where innovative ideas can flourish and pioneering research can be conducted.
Moreover, the intersection of federal funding and scientific inquiry has made significant impacts on the trajectory of neuroscience research. As funding continues to support high-risk, high-reward projects, scientists are empowered to explore unconventional questions, ultimately leading to transformative insights into diseases like Alzheimer’s and Huntington’s. The emphasis on curiosity-driven research, supported by federal investments, will drive forward the development of new therapeutic strategies and interventions for neurodegenerative diseases.
The Future of Alzheimer’s Disease Treatment: An Integrated Approach
Looking ahead, the treatment paradigm for Alzheimer’s disease seems to be shifting towards an integrated, multi-faceted approach. Recent insights from microglial research have underscored the necessity of considering various biological factors in treatment strategies. By combining insights from molecular biology, immunology, and neuroscience, researchers are beginning to draft holistic models that address the complexity of neurodegenerative diseases.
Such integration means that future therapies might not only target the amyloid plaques traditionally associated with Alzheimer’s but also consider the role of inflammation and synaptic pruning conducted by microglia. This comprehensive viewpoint promises to yield treatments that are more effective by addressing the underlying mechanisms driving disease progression. As we continue to understand the interconnectedness of these factors, new therapeutic avenues will likely emerge, ultimately improving outcomes for patients and their families.
The Crucial Role of Neural Circuits in Disease Manifestation
One of the key areas of focus in neurodegenerative disease research is the impact of neural circuits on the manifestation of disorders like Alzheimer’s and Huntington’s disease. The intricate wiring between neurons and how microglia influence these connections can determine the overall health of the brain. By studying these neural networks, researchers can identify critical disruptions that lead to cognitive decline.
The significance of circuits in disease progression highlights the importance of continued exploration into how microglial cells interact with neurons during both healthy and impaired states. Understanding these dynamics is vital to developing therapeutic strategies aimed at enhancing synaptic function and restoring communication between neurons. As the scientific community sheds light on these relationships, we can anticipate advancements in treatments that not only stop disease progression but also promote brain health and longevity.
Collaboration Across Disciplines in Alzheimer’s Research
The complexity of Alzheimer’s disease necessitates a collaborative approach among scientists from diverse fields. This trend towards interdisciplinary cooperation has become increasingly evident in contemporary neurodegenerative disease research, as exemplified by Beth Stevens’ work. By bringing together neuroscientists, immunologists, and geneticists, researchers are creating a comprehensive framework that addresses the multifaceted nature of these conditions.
This collaborative spirit fosters innovation, as diverse perspectives lead to the exploration of unconventional strategies for studying and treating Alzheimer’s disease. For instance, merging insights on the role of microglial cells with genetic studies may uncover novel pathways for intervention. As the scientific community continues to dismantle the silos that previously separated research disciplines, we move closer to effective and transformative treatments for neurodegenerative diseases across the board.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells function as the brain’s immune system, actively monitoring for injury or illness. In Alzheimer’s disease research, studies have shown that these cells can sometimes engage in aberrant pruning of synapses, which disrupts neuronal communication and contributes to disease progression. Understanding their role is crucial for developing potential therapeutic interventions.
How does neuroscience research involving microglia impact Huntington’s disease understanding?
Neuroscience research focusing on microglia is critical to understanding Huntington’s disease, as these immune cells are involved in clearing damaged cells and maintaining brain health. Aberrant microglial activity may lead to neuronal loss, linking this immune response to the pathology of Huntington’s, thus opening new avenues for treatment development.
What advancements have been made in neurodegenerative disease research regarding synaptic pruning?
Recent advancements in neurodegenerative disease research highlight the importance of synaptic pruning, particularly how abnormal microglial activity can lead to the loss of synapses in Alzheimer’s and Huntington’s diseases. By identifying faulty pruning mechanisms, researchers can create targeted therapies that aim to restore healthy brain function.
Why is the study of the brain immune system crucial for Alzheimer’s disease research?
Studying the brain immune system is crucial for Alzheimer’s disease research as it sheds light on the role of microglial cells in disease mechanisms. Understanding how these immune cells contribute to synaptic pruning and neuroinflammation can lead to innovative strategies for early detection and potential treatments for Alzheimer’s.
How does foundational research contribute to neurodegenerative disease treatments?
Foundational research plays a vital role in neurodegenerative disease treatments by uncovering critical pathways and biological mechanisms, such as the role of microglial cells in Alzheimer’s and Huntington’s diseases. This basic science paves the way for translating findings into practical therapies that can improve patient care and outcomes.
What is the significance of biomarkers in neurodegenerative disease research?
Biomarkers are significant in neurodegenerative disease research as they enable early detection and assessment of diseases like Alzheimer’s and Huntington’s. Research focused on identifying microglial-related biomarkers can lead to improved diagnostic tools and targeted therapies that enhance patient management and treatment efficacy.
How do microglial cells influence neurodegenerative diseases beyond Alzheimer’s?
Microglial cells influence several neurodegenerative diseases beyond Alzheimer’s, including Huntington’s disease, by mediating immune responses and neuronal health. Dysfunctional microglial activity can exacerbate neurodegeneration, making them a key target for research aimed at therapeutic interventions across various neurodegenerative conditions.
What impact do NIH grants have on neurodegenerative disease research?
NIH grants significantly impact neurodegenerative disease research by providing essential funding that supports studies like those exploring microglial functions in Alzheimer’s and Huntington’s diseases. This financial backing enables innovations and advances in understanding the complex interactions within the brain’s immune system.
How does curiosity-driven research advance our understanding of neurodegenerative diseases?
Curiosity-driven research advances our understanding of neurodegenerative diseases by allowing scientists to explore novel concepts, such as microglial interactions within the brain. Such research often leads to unexpected discoveries that can reveal new therapeutic targets and enhance current treatment strategies.
What are the future directions for Alzheimer’s disease research involving microglial cells?
Future directions for Alzheimer’s disease research involving microglial cells include exploring their role in neuroinflammation, assessing the impact of targeted therapies on synaptic health, and identifying novel biomarkers. This research aims to develop improved strategies for intervention and personalized medicine approaches for patients.
| Key Points | Details |
|---|---|
| Transformative Research on Microglial Cells | Beth Stevens has reformulated our understanding of microglia, the brain’s immune cells, highlighting their role in Alzheimer’s and other neurodegenerative diseases. |
| Role in Synaptic Pruning | Microglia help in clearing damaged cells and pruning synapses, essential for healthy brain function. |
| Aberrant Pruning Effects | Faulty pruning by microglia can lead to conditions like Alzheimer’s, emphasizing the need for understanding this process in research. |
| Foundation of Future Treatments | Stevens’ research lays groundwork for new biomarkers and treatments for neurodegenerative diseases. |
| Importance of Basic Science | Initial curiosity-driven research has led to significant advancements in understanding neurological diseases. |
Summary
Neurodegenerative disease research is crucial for unraveling the complexities of conditions like Alzheimer’s disease. As illustrated by Beth Stevens’ groundbreaking studies, understanding the role of microglial cells in the brain has opened up new avenues for potential treatments. By focusing on the foundational and curiosity-driven aspects of science, researchers can gain valuable insights into the mechanisms underlying these diseases, ultimately improving the quality of life for millions affected by neurodegenerative conditions.